Nagoya City Science Museum
TOP > Exhibition Guide > Keyword Search > Starting with "W" > water > Water Molecule
Water Molecule

Purpose of Exhibition
There are 100 million kinds of substances on earth.
However, the only 3 states that can easily be observed are solid, liquid and gas [water (ice, vapor)].
We are unable to see phenomena such as the fact that ice (solid) can float on water (liquid). However, most solid materials are heavier than liquid and sink. That is because, in the natural world, water is an eccentric substance.
In this exhibition, the reason for the transforming eccentric behavior of the water is explained with a video and a molecular model.

Additional Knowledge
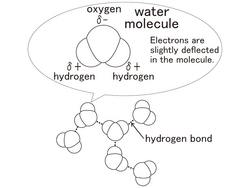
[Water Molecule and Hydrogen Bond]
One molecule of water is made of 2 atoms of hydrogen and one atom of oxygen. A molecule of water is extremely small. In one cup of water, (180ml) there are approximately 6,000,000,000,000,000,000,000,000 molecules of water.
Because the oxygen atoms of the water molecule which has the form of the [<] sign strongly attract electrons, it causes electric polarization. The oxygen atom is slightly negative and 2 hydrogen atoms slightly positive. Therefore, the water molecules electrically strongly attract the nearby water molecules. This is called a [hydrogen bond].
[How does ice mysteriously float on water?]
Ice, whose water molecules are regularly allotted, are structured with more clearance.
Molecules in a substance in liquid state don't follow an allotted order. But in a solid state, the molecule lines are regularly allotted.
However, when molecules in a solid substance other than water are more densely packed together, it becomes heavier than the disordered molecules in the liquid state.
On the other hand, the molecules within the ice are bound in a tetrahedral due to the hydrogen bond with the surrounding 4 water molecules. Therefore, the molecules of ice become arranged with high density and become small, as a result. Ice floats on water. What will happen if ice is heavier than water?In winter, pond fish stay still at the bottom. The water frozen on the surface water below freezing point becomes ice and sinks, and that repeats itself. Ice increases cumulatively from the bottom of the pond and then the entire pond becomes frozen. The fish can survive winter because water is an eccentric.
When applying pressure on the ice, in a way to reduce the volume, this means changing from ice to water. This characteristic cannot be observed with other substances.
[Relationship between Water and Heat]
Specific heat is highCompared to other substances, water's specific heat is high, that is, water requires more heat energy to raise the temperature. Also water neither warms nor cools down easily.
If there is no water, it will be extremely hot during the daytime and very cold in the evening in the desert.
Evaporation heat (Vaporization heat) and heat of solidification is high When changing states from solid to liquid, thermal energy is required.
When watering the road or a garden in a summer evening, temperature cools down when water becomes vapor, because it takes away a lot of heat from the environment.
Melting point and boiling point are extremely highAs an example, you can compare water and hydrogen sulfide at melting point and boiling point. The melting point of water is 0 and the boiling point is 100 degree in Celsius .
Hydrogen sulfide is a component of the smelly and familiar gas to us which can be smelled from craters and at a hot spring. The melting point is -85,5 degree in Celsius , and its boiling point is -60,7 degree in Celsius .
Its molecules are structured similar to water molecules, which consists of one atom of sulfur and 2 atoms of hydrogen. However, there is a considerable difference. Water melting point and boiling point are extremely high.
The strange properties of water requires an enormous amount of energy to split up water molecules which are bound together tightly by hydrogen bond.
[Water Surface Tension Is High]
Other than metals (ex. Mercury) water has the highest surface tension. Surface tension is caused by the mutual attraction of molecules.
Because of the strong hydrogen bond force, surface tension is high.
[Water dissolves different things]
Water constantly circulates while changing its shape on the planet from rain, snow, to rivers and vapor. Water has a strong force to dissolve and carry substances.
For example, in 1 liter of water, 35 g of different substances (Including 24 to 28g of salt) are dissolved. Why can water be used to wash? That's because dirt can be dissolved in water. There is an electric polarization of water molecules. Also in other substances too, there are molecules and ions with minus electricity and plus electricity. Water dissolves substances like this well. Different substances are dissolved in tap water and regular water.
[Ultra pure water] which is made removing something impure has a high capacity to dissolve substances, and is used to clean dirt off high precision machinery, such as computer parts.
Article and illustrations by Keiko Ishida, curator
