Nagoya City Science Museum
TOP > Global Warming > What is the ozone hole?
What is the ozone hole?
The ozone hole has become a household term.
Let's just revise why it occurs.
"How was the ozone hole formed?"
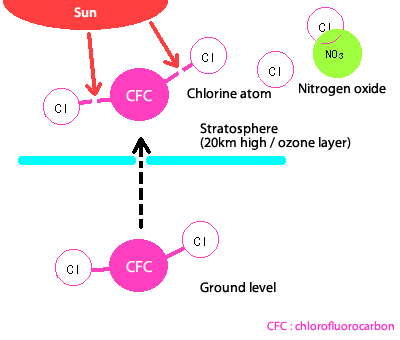
Chlorofluorocarbons are released by industrial activities including washing and cooling.
The sun's light is not strong enough to cause their decomposition until they get very high up in the atmosphere, and they do not dissolve in rain so they disperse and rise into the air.
Once they get to a height of about 20km, they are broken up by the strong sun light, and chlorine atoms are liberated.
If they remained in this form, these chlorine atoms would destroy ozone, and ozone holes would arise all around the planet.
However, under normal conditions, these chlorine atoms are captured by nitrogen oxides, and float around as stable substances that do not destroy ozone.
"Then why does an ozone hole form?"
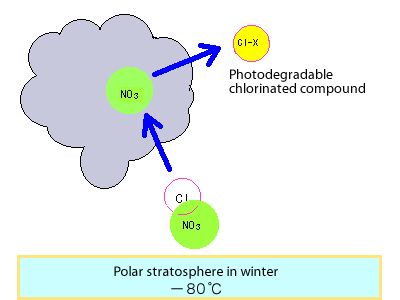
Now we change locations to the north and south poles in winter. Normally, at heights of 20km, there is very little water vapour so clouds cannot form.
However, in these periods, in these regions, it becomes extremely cold (below -80°C), and special clouds (Polar Stratospheric Clouds: PSCs) form.
If one of these cloud particles comes into contact with one of these nitrogen oxide compounds that has captured a chlorine atom, the nitrogen oxide is captured in the cloud particle and the chlorine atom is thrown off as a molecule (chlorine molecule or hydrogen chloride).
The cloud particles containing the nitrogen oxides gradually fall down, leaving the chlorine molecules and hydrogen chloride behind.
These molecules are different to the previously mentioned stable substances, and easily release chlorine atoms when exposed to the sun's light.
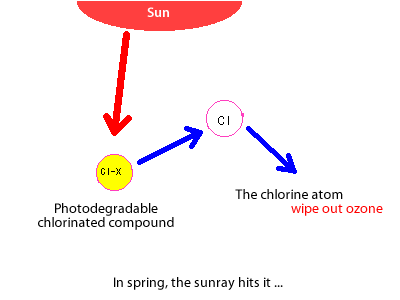
The molecules created in this way in winter then wait for the spring sunlight to return and liberate their chlorine atoms in a single burst.
At this point the nitrogen oxides are almost all gone, and ozone continues to be destroyed causing an ozone hole.
This is why the ozone hole appears in high-latitude regions in spring.
Air comes in from low latitudes and the ozone hole continues until the nitrogen oxides in this air capture the chlorine atoms.
This is only a rough explanation. The place where the ozone hole forms is difficult to observe, so the mechanisms are still not understood well enough.
This is true not only for the ozone hole, but for all fields of research into the planet's environment. The appearance of many new scientists who will clarify these things in the future is eagerly awaited.
